PUBLICATIONS

Circulating tumor DNA after neoadjuvant chemotherapy is a better prognostic test than Residual Cancer Burden inpatients with triple negative breast cancer and residual tumor
Circulating tumor DNA (ctDNA), a plasma-based biomarker, reveals real-time data about disease/treatment progression. We have previously shown that ctDNA detection after NAC and prior to surgery signals poor prognosis in TNBC patients using an academic hospital-based tumor bespoke assay. To validate and extend these results, we performed ctDNA measurements at 4 time points in non-pCR TNBC patients participating in our TRICIA study (NCT04874064).

The Prognostic Value of Circulating Tumor DNA in Triple Negative Breast Cancer Patients with Incomplete Response to Neoadjuvant Chemotherapy
The most aggressive form of breast cancer is triple negative breast cancer (TNBC), so called because these tumors do not express estrogen, progesterone, or HER2 receptors. About half of TNBCs treated with neoadjuvant chemotherapy (NAC) will have a residual tumor at surgery (non-pathological complete response or non-pCR), signaling chemoresistance and poor prognosis. Further adjuvant chemotherapy (Xeloda) results in improved survival in about 15% of non-pCR patients. Circulating tumor DNA (ctDNA), a plasma-based biomarker, reveals real-time data about disease/treatment progression.

CADTH Symposium 2023: PERSONALIZE MY TREATMENT (PMT): A CANADIAN CANCER PATIENT REGISTRY
Exactis presented our PMT Databank at the 2023 CADTH Symposium in Ottawa in May 2023. Our pan-Canadian network of hospital sites across the country helped us enroll patients and collect longitudinal real-world data (RWD) in a standardized secured de-identified online database. To date, we have close to 9,000 cancer participants.
IARC Nov 8-10 conference 2022
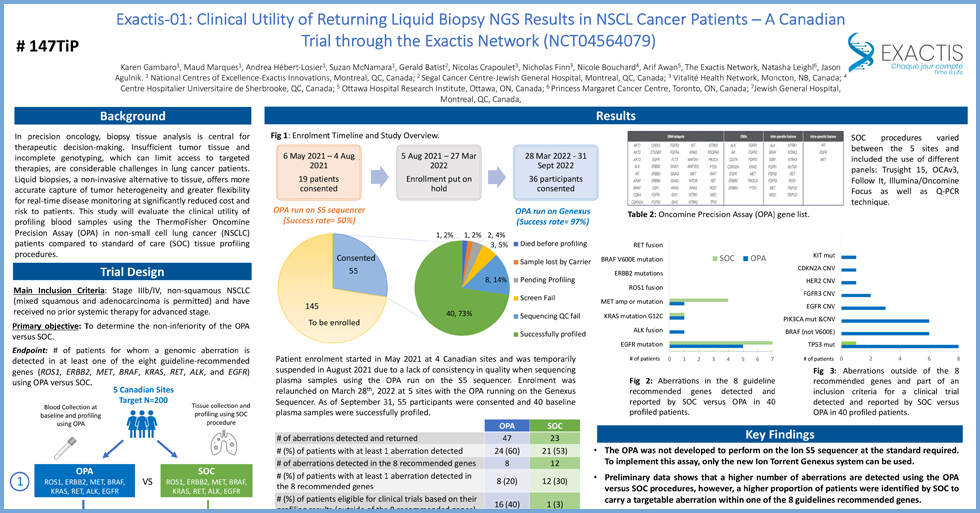
Exactis’ PMT registry offers invaluable insights into disease impacts, disparities, and treatment patterns. PMT, a standardized digital cancer registry, spans 16 hospitals across 5 Canadian provinces. This comprehensive data, inclusive of demographics, diagnosis, treatments, and biomarkers, underscores the registry’s potential to enhance our understanding of oncological trends in Canada, ensuring more accurate evaluations of clinical benefits and treatment gaps in the real-world setting.